CHEM60112Y
化学代考 The use of molecular models is allowed A table of physical constants and conversion factors is attached A periodic table is attached…
The use of molecular models is allowed
A table of physical constants and conversion factors is attached
A periodic table is attached
PERSONALISED LEARNING UNIT (CHEM60112 PART B) RESIT PAPER
Answer ANY SIX questions.
Submit each question answer as a SEPARATE upload.
Each upload should be submitted as a file named as:
StudentId_CourseCode_QuestionNumber, e.g. 12345678_CHEM60112Y_Q1
The total mark for this paper is 150 marks
Each question is worth 25 marks
-
CONTEMPORARY f-ELEMENT CHEMISTRY 化学代考
Answer ALL parts.
(a) (i) Use valence electronic configurations to explain which lanthanide ions have the most well-developed +2 oxidation state chemistry.
(3 marks)
(ii) Explain why, with the exception of No(II), actinide cations in the +2 oxidation state are all less stable than the lanthanide(II) cations discussed in
part (i).
(2 marks)
(iii) CO binds readily with [U{C5H4(SiMe3)}3], but CO does not bind readily with [Nd{C5H4(SiMe3)}3]. Explain these observations.
(2 marks)
(iv) Explain why the relative strength of metal-carbon back-bonding for the carbonyl ligands of [Zr(C5H5)2(CO)2] and [U{C5H4(SiMe3)}3(CO)] cannot
be determined by comparison of the C≡O stretching mode frequencies in their infrared spectra.
(3 marks)
(b) (i) The monometallic uranium complex [U{C8H6(SiMe3)2-1,4}(C5Me5)] reacts with CO to give a dimetallic uranium complex. Predict two possible identities for the product of this reaction and briefly explain how the results from two different experimental techniques could distinguish the products from each other.
(4 marks)
(ii) Identify compounds A-F, using the balanced equations and characterisation data provided below.
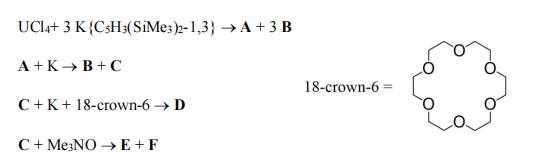
A: Magnetic data showed 2 unpaired e– . B: Colourless diamagnetic solid, sparingly soluble in THF, no 1H NMR signals. C: Magnetic data showed 3
unpaired e– , EPR signal observed. D: Magnetic data showed 4 unpaired e– . E: Magnetic data showed 1 unpaired e– , EPR signal observed. F: Colourless diamagnetic gas at 298 K; 1H NMR: δ 2.12; 13C NMR: δ 47.46.
(6 marks)
(c) (i) Predict the most likely products that would form when the uranium nitride complex [Na(12-crown-4)2][N≡U{N(CH2CH2NSiiPr3)3}], G, reacts with
half an equivalent of diiodine, I2.
(2 marks)
(ii) G reacts with one equivalent of BPh3 to give a single product H. Predict the identity of H, providing your reasoning. State how you would expect the frequency of the U≡N stretching mode in the IR spectra of G and H to differ.
(3 marks)
-
CATALYTIC ASYMMETRIC SYNTHESIS
Answer ALL parts.
(a) The reaction sequence shown below is used in the synthesis of (S)-metoprolol, (S)-3.
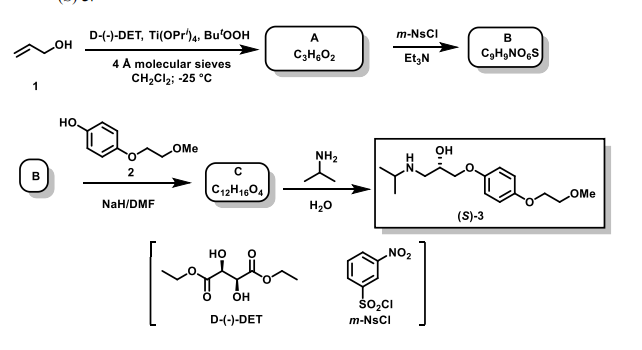
(i) Use the Sharpless mnemonic to suggest a structure for A; label any stereogenic centres generated in this reaction with their predicted absolute
stereochemistry for the major product.
(6 marks)
(ii) Suggest a structure for B, noting its absolute stereochemistry.
(2 marks)
(iii) Suggest a structure for C and draw a mechanism for the conversion of B into (S)-3.
(5 marks)
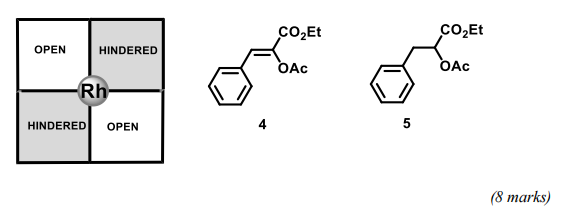
(b) Using the quadrant diagram for (R,R)-[Rh(DiPAMP)(COD)]BF4, shown below, rationalise why hydrogenation of alkene 4 proceeds in high enantiomeric excess (90% ee). Using this model predict the major enantiomer of 5 resulting from the reduction of 4 under these conditions; assign the absolute configuration to this product.
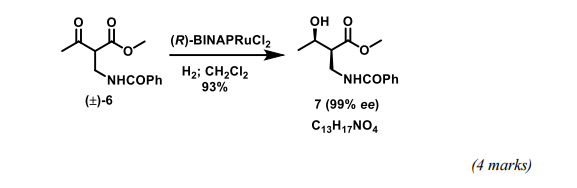
(c) Catalytic hydrogenation of (±)-6 with (R)-[Ru(BINAP)Cl2] affords (2S,3R)-7 as the major product in 93% yield and with 99% ee. Careful analysis of the product mixture from this reduction also indicates the presence of three other alcohols, all of which have the molecular formula C13H17NO4. Suggest structures for these minor by-products and comment upon the factors responsible for the stereochemical course of the reaction.
-
POLYMER MATERIALS 化学代考
Answer ALL parts.
(a) (i) Describe what happens to the temperature of an elastomer when it is stretched and explain this using a thermodynamic argument that discusses the changes in the entropy of an elastomer on stretching.
(5 marks)
(ii) How does the elastic behaviour of an elastomer differ from that of a glassy material and how can it be explained in molecular terms?
(5 marks)
(b) The figure below shows tensile stress-strain curves for a sample of polystyrene (PS) and a sample of high impact polystyrene (HIPS).
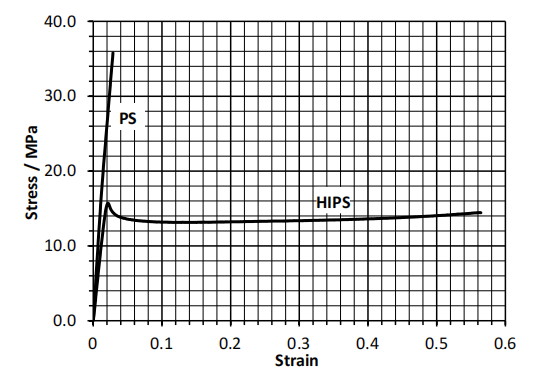
Using the figure, for each material determine the Young’s modulus, the tensile strength and, if applicable, the yield stress. Discuss, with explanation, which of these materials shows the higher toughness.
(10 marks)
(c) Explain what is meant by the term life cycle assessment and discuss how such assessments may enable more sustainable use of polymers.
(5 marks)
-
APPLIED OPTICAL SPECTROSCOPY 化学代考
Answer ALL parts.
(a) Evaluate the use of an array detector in a conventional FTIR spectrometer.
(5 marks)
(b) The image below shows the infrared spectrum of methane in the gas phase at a resolution of 4 cm–1 . It was obtained from a low-pressure sample in a 10 cm path length and took 1 min to collect from the co-addition of 16 scans.
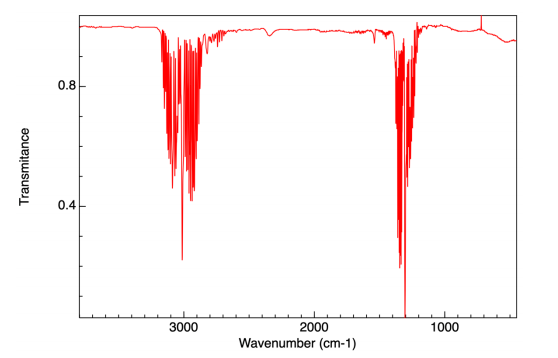
(i) Taking values from the spectrum, calculate the absorbance values for the highest intensity bands in each of the two modes shown. Comment on the magnitude of the relative error in the resulting absorbance values.
(3 marks)
(ii) Given that the concentration is effectively 0.3 mM (in the gas-phase), calculate the molecular cross-section in cm2 molecule–1 for the band around 3000 cm–1 . Comment on the magnitude of the value you obtain.
(4 marks)
(iii) For a particular purpose, a spectrum at 0.5 cm–1 resolution is needed. Describe the experimental challenges of achieving this resolution in an FTIR spectrometer. 化学代考
(3 marks)
(c) When ethanol adsorbs on a metal surface such as copper, reflection-absorption infrared (RAIR) spectra reveal that an ethoxy (CH3CH2O–) species forms with a C–O bond perpendicular to the surface and that both carbon atoms retain their sp3 hybridisation. Explain how these facts might be established from the RAIR spectra.
(10 marks)
-
MAIN GROUP REAGENTS IN SYNTHESIS
Answer ALL parts.
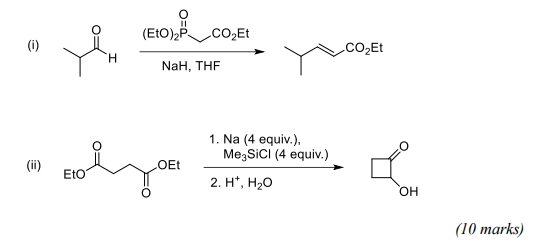
(a) Give mechanisms for BOTH of the transformations shown below, and explain the role of the reagents containing phosphorus and silicon.
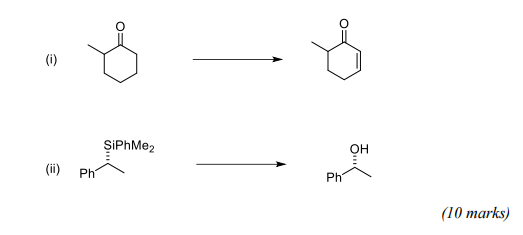
(b) The following transformations involve the chemistry of compounds containing silicon, sulfur, selenium or phosphorus. For BOTH of the transformations, give reagents and mechanisms for the reactions involved.
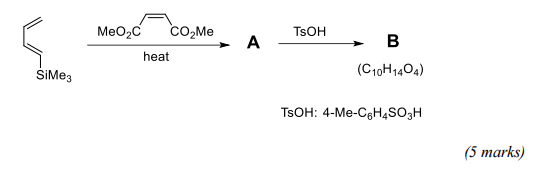
(c) Identify the intermediate A and product B in the following reaction sequence, and give mechanisms for both steps in the sequence.
-
SURFACE ANALYSIS 化学代考
Answer ALL parts.
In order to understand the interaction of oxygen with silver surfaces, a single crystal of silver is prepared by sputtering and annealing and subsequently exposed to molecular oxygen (O2) inside an ultrahigh vacuum chamber. A series of surface science techniques are then used to study the crystal.
(a) A temperature programmed desorption (TPD) experiment is performed and produces the following result:
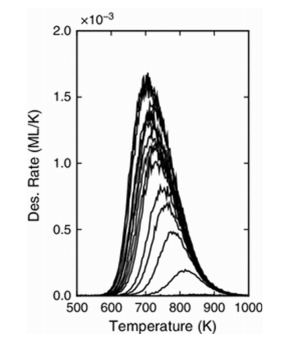
(i) What is the reaction order of this desorption and what does that tell you about the adsorption of oxygen onto the silver surface?
(3 marks)
(ii) Why is TPD often performed on single crystals? What would happen if a sample of silver nanoparticles were used instead?
(3 marks)
(b) Low Energy Electron Diffraction (LEED) patterns are taken of the pristine surface before oxygen exposure (A), after a short oxygen exposure (B) and a long oxygen exposure (C). The patterns are shown below:
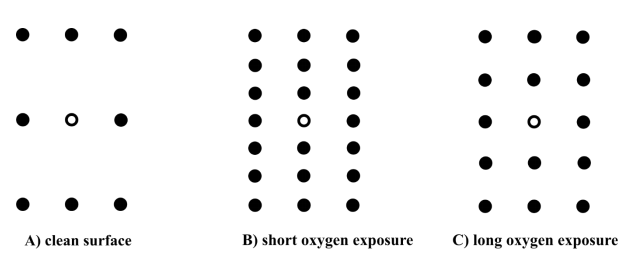
(i) What are the Miller indices of the pristine surface?
(2 marks)
(ii) Identify the oxygen-induced reconstructions in patterns B and C and express them in Wood’s notation.
(5 marks)
(iii) Assuming 1 adsorption site per surface unit cell – what is the oxygen coverage (in terms of % of a monolayer) of the reconstructions in patterns B
and C?
(4 marks)
(c) (i) Assuming a sticking coefficient of 1, how long would it take to produce the oxygen coverage seen in LEED pattern C? The unit cell area for the
unreconstructed surface is 0.235 nm2.
Oxygen pressure = 1×10−6 mbar
Temperature = 298 K
(6 marks)
(ii) A total oxygen exposure of 240 Langmuirs was required to produce pattern C. From your previous answer, determine the sticking coefficient of the
silver surface.
(2 marks)
-
INDUSTRIAL SEPARATIONS 化学代考
Answer ALL parts.
(a) (i) Explain the difference between an absorption process and an adsorption process in the context of industrial separations.
(5 marks)
(ii) Discuss ways in which an adsorbent may be regenerated after being used in an adsorption process.
(5 marks)
(b) (i) At a temperature of 80C and a pressure of 1 atm, the saturated vapour pressures of n-hexane and n-heptane are 1.43105 Pa and 5.66104 Pa, respectively. Calculate the vapour-liquid equilibrium ratio (K-value) in each case, assuming Raoult’s law applies. Hence calculate the mole fractions of each component in the liquid and vapour phases for a mixture of these hydrocarbons at this temperature and pressure.
(5 marks)
(ii) The figure on the next page shows the temperature-composition (T-y-x) phase diagram for a mixture of n-hexane and n-heptane at a pressure of 1 atm. If you wish to distil a mixture of n-hexane and n-heptane with mole fraction of n-hexane, xhexane = 0.1, how many equilibrium stages (theoretical plates) are required to achieve a distillate with xhexane >0.7? How many equilibrium stages are required to achieve a distillate with xhexane >0.85? 化学代考
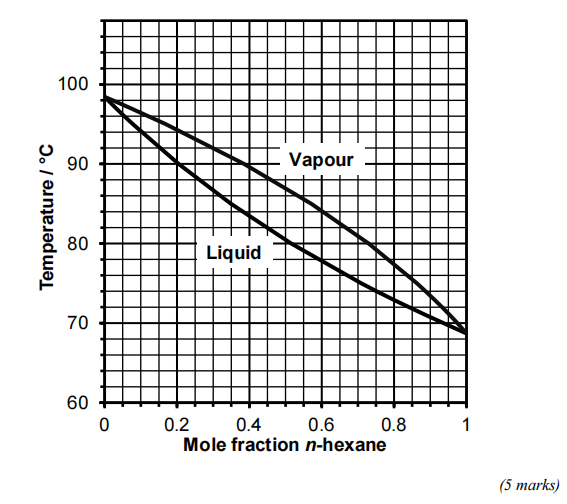
(c) Explain what is meant by the term azeotrope and discuss ways of breaking an azeotrope.
(5 marks)
-
SOLID STATE NMR: PRINCIPLES AND APPLICATIONS
Answer ALL parts.
(a) (i) The table on the next page shows NMR parameters for the first 34 elements of the periodic table. Choose one spin-half nucleus and three quadrupolar nuclei, each with a different nuclear spin. In each case sketch the nuclear energy level splitting expected in the presence of a magnetic field, including both the Zeeman interaction and all quadrupolar interactions.
(5 marks)
(ii) For each of the same nuclei chosen in part a(i), using information in the table on the next page, determine if the nuclei will be easy or difficult to record solid-state NMR spectra. Give your reasoning. 化学代考
(5 marks)
(b) (i) Describe, using diagrams where necessary, the line-broadening mechanism that affects the solid-state NMR spectrum of quadrupolar nuclei.
(5 marks)
(ii) Describe line narrowing techniques suitable for solid-state NMR of quadrupolar nuclei and sketch some illustrative spectra that you might expect for such nuclei.
(5 marks)
(iii) The figure below shows on the left the 23Na MAS NMR spectrum of sodium cations in the mineral narsarsukite. On the right is the structure of narsarsukite with a representation of the electric field gradient (EFG) on a sodium cation within the titanosilicate framework structure. Determine the approximate 23Na isotropic chemical shifts, iso, and asymmetry parameters, of these sodium cations and comment on the shape of the EFG.
(5 marks)




