CENG0004 Thermodynamics Coursework 1
热力学代写 A frictionless piston-cylinder system shown in Figure Q1 is exposed to atmospheric pressure. The piston mass is 100 kg and has an area…
Q1) A frictionless piston-cylinder system shown in Figure Q1 is exposed to atmospheric pressure. The piston mass is 100 kg and has an area of 0.25 m2 . The ideal gas is in the cylinder is at 300 K and has a volume of 0.15 m3 at the initial state. The gas has a constant volume heat capacity,C*v , of 29 J/(mol K). Heat can be added to the gas through a built-in electric heater inside the cylinder but otherwise the piston and cylinder are well insulated. Answer the following after 12.5 kJ of energy is added to the gas using the electric heater:
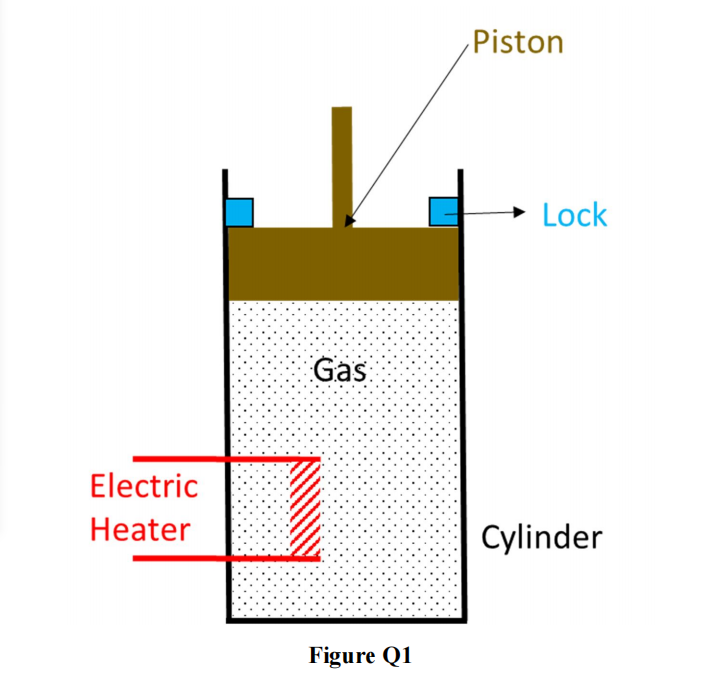
(a) If locks placed at the initial position of the piston prevent it from rising, what will be the final temperature and pressure of the gas?
[5]
(b) If the piston is allowed to move freely, what will be the final temperature and volume of the gas? 热力学代写
[10]
Q2) A piston-cylinder system is built to elevate weights between two levels by attaching a pan on top of the piston. The new system, which is exposed to atmospheric pressure, is shown in Figure Q2. The pan can be elevated by electrically heating the air contained in the piston-cylinder, and the pan can be lowered by opening a valve at the side of the cylinder, allowing the air in the cylinder to slowly escape. Once the pan is back to the lower level, a small pump evacuates the air remaining in the cylinder and replaces it with air at 25°C and a pressure just enough to support the lift. The cycle can then be repeated.
There is no heat transfer between the piston, cylinder, and the gas; the weight of the2 piston and the pan is 3000 kg; the pan (and the piston) has a surface area of 3 m2 ; and the volume of air in the cylinder when the pan is at its lowest level is 20 m3 . The air in the cylinder is assumed to be an ideal gas with C*p = 30 (J/molK).
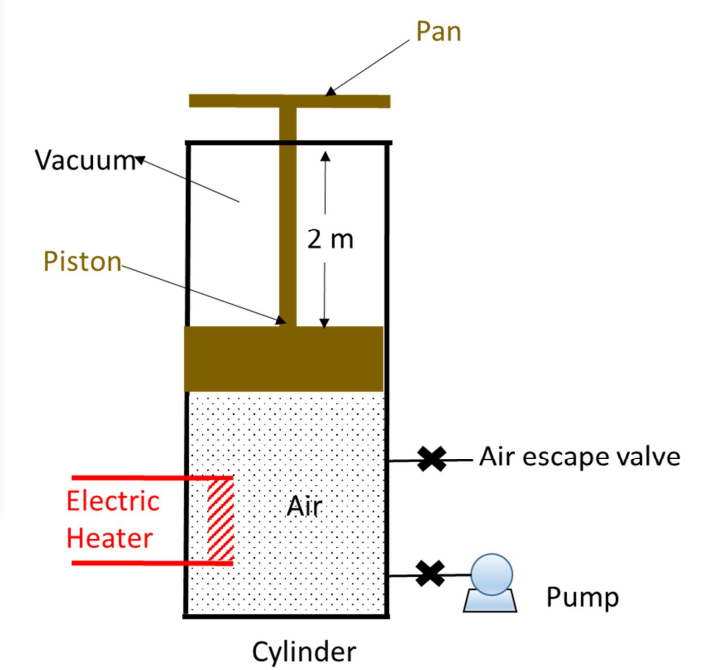
Figure Q2
(a)What is the pressure of air during the process of raising and lowering the pan?
[5]
(b)How much heat must be added to the air during the process of raising the pan by 2 m, and what is the final temperature of the gas?
[10]
(c)What fraction of the heat added is used in doing work, and what fraction is used in raising the temperature of the gas?
[10]
(d)How many moles of air must be allowed to escape in order to lower the pan to its original position; i.e. 2 m lower?
[10]
Q3) If the system depicted in Q2 is to be designed such that the pan ascends and descends at a rate of 0.1 m/s, and is to rise a total of 2 m; 热力学代写
(a)At what rate should heat be added to the cylinder during the ascent?
[10]
(b)How many moles per second of air should be removed from the cylinder during the descent?
[10]
Q4) A passenger plane is flying at an altitude of 40,000 feet. Outside air temperature is−55 ºC and the pressure is 0.2 bar. Fresh air will need to be compressed to 1 bar and heated to 25°C before being introduced into the cabin. The plane’s cabin has a volume of 120 m3 . Calculate the cost of refreshing the cabin air every 1 minute if energy costs £0.78 per kW hr. The air is assumed to be an ideal gas with C*p = 30 (J/molK).
[10]
Q5) Helium is a monatomic gas used for filling flying balloons. Helium gas is being withdrawn at a rate of 2 g/s from a 0.35 m3 cylinder. Initially, the pressure of the gas in the cylinder is 12 bar and its temperature is 310 K. The cylinder is well-insulated and its temperature does not change during the gas emptying process. What will be the temperature and pressure of the gas in the cylinder after 3 minutes? What will be the rate of change of the helium gas temperature at this time? Assume helium is an ideal gas with a constant heat capacity.
[10]
Q6) How many kilograms of liquid water at 2.5 MPa and 25°C should be mixed with 500 kg superheated steam at 3.0 MPa and 500°C to produce saturated steam at 2.25 MPa with 80% quality; i.e. vapour content? 热力学代写
[10]



